AviClear Launches in the UK & Ireland
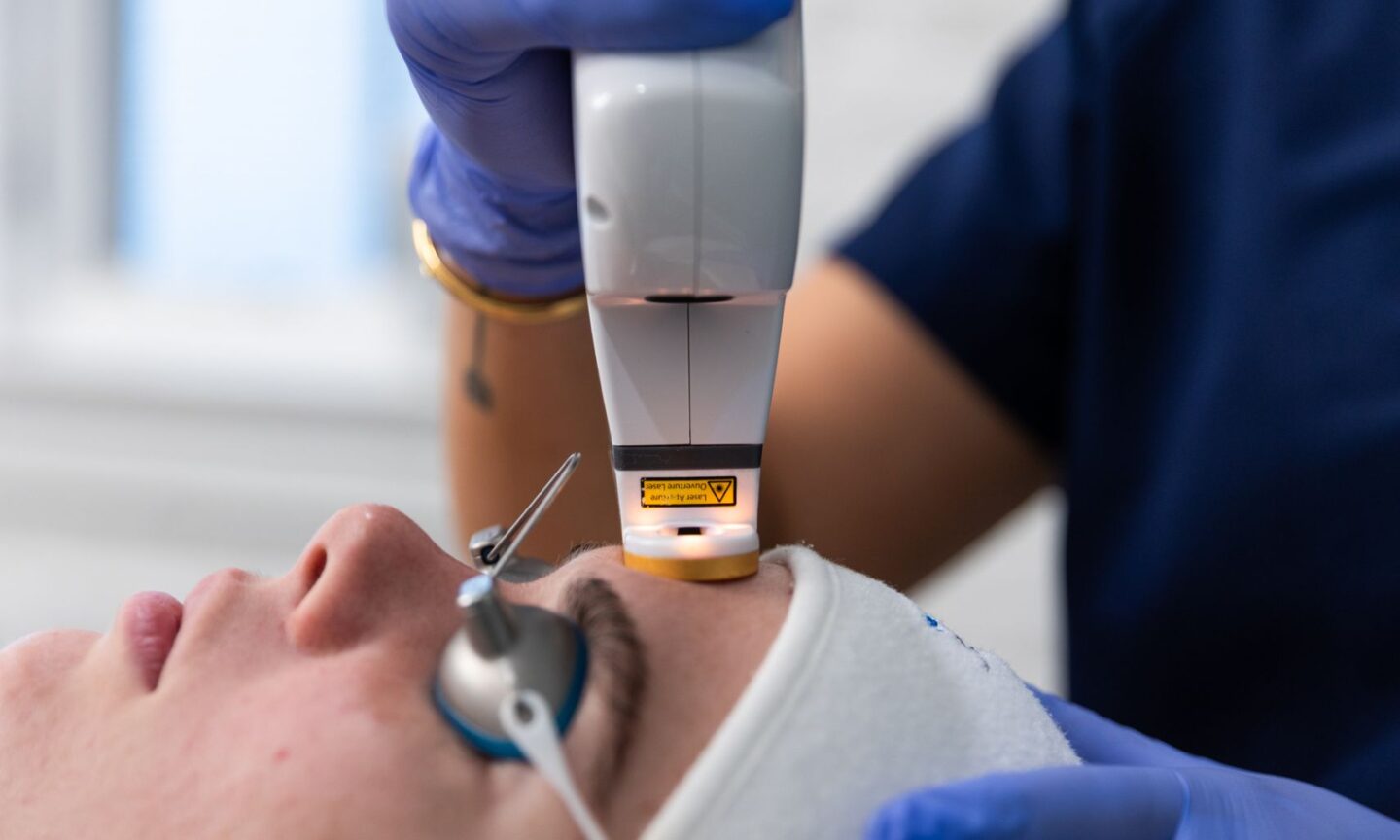
After a successful rollout in the US and Canada, aesthetic device company Cutera Inc. has announced a limited commercial release of AviClear for the UK & Ireland markets.
AviClear is the first FDA-cleared laser for long-term treatment of inflammatory acne vulgaris. Suitable for all skin tones, AviClear selectively targets and suppresses the sebaceous glands, eliminating acne at the source, offering a durable and prescription-free option for patients and providers.
“We are excited to be offering this ground-breaking AviClear treatment to physicians and patients in the UK and Ireland,” said Sam Keene, Cutera regional leader UK and Ireland, “Based on feedback from our early adopters, we are confident that patient demand and clinic installations will continue to grow over the next few months and that the affordability, efficacy, and durability of AviClear will establish it as the gold standard of care for acne sufferers.”
As the first 1726nm laser to be introduced to the market, AviClear continues to challenge the status quo in the acne landscape. In just three 30-minute sessions, 90% of patients experienced visible improvement in their acne six months after their final treatment. According to 12-month clinical data, improvement increases to 92%, confirming long-term efficacy of acne clearance and skin quality over time.
To find your nearest AviClear treatment provider, visit aviclear.com.